Fenusulin®
- Home
- Nutritional Ingredients
- Fenusulin®
Fenusulin® Technical Brochure INQUIRE
| Botanical name | Trigonella foenum-graecum L. |
| Family | Fabaceae |
| Common Name | Fenugreek |
| Part used | Seed |
Fenusulin® is 4-hydroxyisoleucine standardized extract of Fenugreek. It has a long history of medical uses in Ayurveda and Chinese Traditional Medicine. Most applicable part of Fenugreek is its seed.
Phytoconstituents:
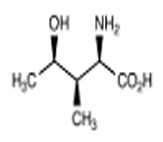
T. foenum-graecum seed is good source of protein (20-30%), fat (6.53%) and crude fibre (6.28%).[1] It mainly contains 4-hydroxyisoleucine, trigonelline, galactomannan with flavonoids, carotenoids, coumarins, proteins, saponins, and lipids.[2]
 |
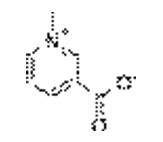 |
| 4-hydroxyisoleucine | Trigonelline |
Clinical indication:
Fenugreek is commonly consumed as a condiment and used medicinally as a galactagogue by nursing mothers to increase inadequate breast milk supply.[3] Seed extract decreases of diabetes symptoms such as polydipsia, polyuria, urine sugar, renal hypertrophy and glomerular filtration rate. T. foenum-graecum seeds showed hypoglycemic and hypocholesterolemic effects in type 1 and type 2 diabetes patients.[2] 4-hydroxyisoleucine proved to have antidepressant-like effects.[4]
Safe to consume:
Fenugreek spice, oleoresin, extract have been listed as GRAS (21 CFR part 182) by FDA. Fenugreek has been used for several centuries and no adverse side effects have been noted.[5]
References:
- Atefeh Sheikhlar. 2013. Trigonella foenum-graecum L. (Fenugreek) as a medicinal herb in animals growth and health. Sci Int. 1(6): 194-198
- Kulkarni et al. 2012. Antidiabetic activity of Trigonella Foenum-graecum L. seed extract (IND01) in neonatalstreptozotocin-induced (N-STZ) rats. Diabet Croatica 41(1): 29-40
- Sreeja et al. 2010. In vitro estrogenic activities of fenugreek Trigonella foenum graecum seeds. Indian J Med Res 131: 814-819
- Gaur et al. 2013. Antidepressant-like effect of 4-hydroxyisoleucine from Trigonella foenum graecum L. seeds in mice. Biomed and Aging Path. 2(3): 121-125
- FDA. 1996. http://www.fda.gov/ohrms/dockets/dockets/95s0316/rpt0006_01.pdf



